Oxidative stress in cell culture: getting the most from your growth factors
Andrew D. Schaefer, MBA, Manager of Analytical Method Development & Validation, Biopharmaceutical Raw Materials, Eurofins BioPharma Product Testing, Andrew.Schaefer@bpt.eurofinsus.com

Cell and gene therapies (CGT) are one of the fastest growing modalities, and the Eurofins BioPharma Product Testing (EBPT) Raw Materials network of laboratories is acutely aware of the challenges our customers face in this area. In support of industry efforts, the EBPT Raw Materials CGT team has dedicated its expertise and time to collaborating with our partners and the Pharmacopeia to develop new testing standards and innovative approaches to solve unique problems. Beyond supporting new Compendial chapters, the Raw Materials team has published work on cell culture applications of Interleukin-2 Stability in the American Journal of Analytical Chemistry and N-acetyl-L-cysteine and N,N’-diacetyl-L-cystine Stability in Cell & Gene Therapy Insights. A third installment in this effort, titled The Impact of EDTA and Selenite on the Stability of Insulin in Cell Culture Media, was recently published in the International Journal of Biochemistry Advances.
This latest work investigated two common cell culture supplements, disodium edetate (EDTA) and sodium selenite (Se), and their impact on the stability of a ubiquitous peptide hormone, Insulin. Modeled in the classical Dulbecco’s Modified Eagle Medium and Roswell Park Memorial Institute 1640, it explored whether the antioxidant and radical-scavenger properties of EDTA and Se would decrease the levels of peroxide in media. Curiously, this work found that Se did not improve peroxide levels in media despite previous literature reporting antioxidative properties. Mechanisms were hypothesised for this phenomenon, and the work presents proposed supplementation levels for users to reduce peroxides in source media and subsequently oxidative stress on their cells.
The Eurofins BPT Raw Materials laboratory in Lancaster, PA, is one of the largest single raw materials laboratories in the world, with a network of companion laboratories in North America and abroad designed to deliver fast and reliable quality control testing to CGT clients and traditional manufacturers. Eurofins BPT provides a one-stop-shop for full GMP raw materials testing, covering chemistry, biochemistry, microbiological and viral services. Eurofins BPT’s Compendial monograph testing services offer the largest array of turnkey QC testing in the industry, with multiple shifts of staff ready for samples, day or night. A dedicated cohort of scientists can support a variety of method development and validation needs, and implementation of methods for non-Compendial materials is complemented by over 80 platform methods designed to get clients’ materials ready for GMP QC testing as fast as possible. Contact us to learn more. Or visit EBPTRawMaterialsTestingForCGT.
How effective is the cleaning process of my medical device?
Melanie Mitterreiter, PhD, Consultant, MelanieMitterreiter@eurofins.com;
Anja Friedrich, Head of Consulting, Eurofins Medical Device Consulting, AnjaFriedrich@eurofins.com

The manufacturing process of various medical devices – from surgical instruments to blood contacting devices and implants – includes one or more cleaning steps in order to remove particulate matter as well as chemical or biological contaminants.
The cleaning process may include different techniques (e.g. ultrasonic baths, floating and rinsing, wiping, plasma cleaning, etc.), and cleaning agents, leading to possible contamination. The device design itself (e.g. crevices, clamps or joints, rough and irregular surfaces) may also be a source of risk.
Within the scope of the biological risk assessment, according to ISO 10993-1, the efficiency of the cleaning process has to be evaluated by the manufacturer. The first step within the development of validating the cleaning process is an analysis of potential contaminations and a detailed description of the cleaning process and the chemistry used. In the second step, suitable testing methods are chosen, and the required sample size, as well as the acceptance criteria for the individual medical device, are determined. The Eurofins Consulting team offers a new service that supports manufacturers by preparing the cleaning validation strategy, considering the intended use of the device, as well as applicable guidelines and regulations, such as ISO 19277, DIN/TS 5343, ASTM F3127, AAMI TIR 42.
Our consultants further offer gap analysis of pre-existing validation plans. The team also supports medical device manufacturers in case of any change (to the device itself or to the manufacturing and cleaning processes) by evaluating their impact on the cleaning validation. Eurofins Consulting not only offers cleaning validation services, but also cleaning services since the recent acquisition of Eurofins Inpac Medizintechnik and Eurofins Steripac in Germany. These sites offer in-house solutions for cleaning (amongst other services like sterilization or packaging) with highly effective automated qualified equipment and validated procedures within their ISO class VII clean rooms. For more information about services for medical device documentation and evaluation, visit: www.eurofins.de/medical-device-consulting/ More information for in-house cleaning can be found here: www.inpac-medizintechnik.de/ and https://steripac.com/
A comprehensive testing solution for mycoplasma comparable validation
Willard Sun, Technical Sales Manager, Eurofins BioPharma Product Testing Shanghai, willard.sun@cpt.eurofinscn.com

Mycoplasma is a common type of cell line contamination that may affect the manufacture of bioproducts, so it should be carefully controlled throughout the bioprocess. Contamination with mycoplasma is traditionally detected by direct or indirect culture-based methods. However, these methods will take several weeks to yield results, so the step is increasingly seen as a bottleneck for releasing bioproducts.
There is therefore a need for rapid alternative methods with a comparable sensitivity, specificity and species range, and the NAT method is becoming more and more accepted by biopharma companies in this regard. Several commercially available kits for mycoplasma detection exist on the market; these kits can detect less than 10 CFU of the most frequently encountered mycoplasma contaminants in mammalian cell cultures.
Before these kits can be used as a routine test in the production of cell culture-based biologicals, validation according to the pharmacopeias is a must, especially the European Pharmacopoeia and Japanese Pharmacopoeia.
Eurofins BPT Shanghai collected all mycoplasma needed in the Ph. Eur. <2.6.7> and successfully set up a platform to help clients perform mycoplasma detection and qPCR method validation, including the culture-based method comparation component. Eurofins BPT Shanghai Laboratory specialises in mycoplasma comparable validation services, which provide customers with a more comprehensive testing solution and more accurate test results in a shorter period of time. EBPT Shanghai can support the validation of all kinds of NAT method mycoplasma testing kits, such as:
Specificity: The ability to correctly determine mycoplasma in the presence of other components (e.g., foreign bacterial contamination, cell matrix, and host cells).
Detection limit: The limitation to detect all kinds of mycoplasma. In Ph. Eur. <2.6.7>, it is required that each dilution of each mycoplasma has 24 detection data, and the positive rate of detection needs to meet more than 95% in order to be used as the detection limit.
Robustness: Whether the measurement results are affected when the measurement conditions are slightly changed.
For more information, contact: BPTSH@cpt.eurofinscn.com
Eurofins Discovery presents obesityLITE Panel Fit-for-Purpose Solutions
Lakshmi Anantharaman, Director of Screening, lakshmi.anantharaman@discovery.eurofinsus.com;
Luke Oostdyk,Ph.D., Associate Study Director, luke.oostdyk@discovery.eurofinsus.com; Eurofins Discovery LeadHunter Services US
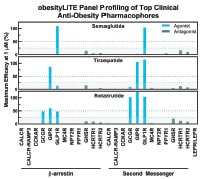
Obesity is a serious, chronic disease currently affecting about 39% of the global population, a figure that is expected to rise to over 50% by 2035, according to a recent report from the World Obesity Atlas (WOA). Single GLP-1R agonist, Semaglutide (Novo-Nordisk), recently gained market interest as an approved drug for obesity management. Now many pharmaceutical companies are pursuing a strategy of simultaneously targeting multiple receptors for additive benefits, the most attractive of these being dual GLP-1R/GIPR agonist, Tirzepatide (approved drug developed by Eli Lilly), and triple GLP-1R/GIPR/GCGR agonist, Retatrutide (in clinical development by Eli Lilly).
With anti-obesity target combination therapies quickly gaining market interest, Eurofins Discovery LeadHunter® Services now includes the obesityLITE panel, featuring a focused set of cell-based assays ideal for testing the functional activity of desired therapies against multiple disease-relevant targets in one convenient screen. This one-of-a-kind assay panel enables accelerated assessment of potency, selectivity, and mechanism of action characterisations through functional dose response profiling of small and large molecule libraries. obesityLITE includes 25 assays targeting leptin receptor and G-protein-coupled receptors (GPCRs) implicated in metabolic regulation, including GLP-1R, GIPR, GCGR, MC4R, CCKAR, amylin, neuropeptide Y, ghrelin, and orexin receptors. The panel offers profiling in GPCR β-arrestin or second messenger assays in agonist or antagonist modes, enabling the detection of any biased compounds. The graphic highlights an example of the effectiveness of the obesityLITE panel in profiling for multi-receptor targeted therapies.
Eurofins Discovery LeadHunter Services’ robust platform offers the flexibility to create personalised screening solutions by combining panel screen with ad hoc counterscreen assays from a comprehensive set of GPCR assay services. The obesityLITE panel was launched at a cost-effective introductory panel fee so Eurofins Discovery LeadHunter Services can contribute to accelerating weight loss programme goals. Learn more at eurofinsdiscovery.com
Eurofins BPT Les Ulis at the forefront of inhalation products testing
Marine Archambault, Group Leader, MarineArchambault@eurofins.com; and
Mathieu Van Schel, Business Unit Manager, mathieuvanschel@eurofins.com; Eurofins BioPharma Product Testing, Les Ulis (France)

Inhalers are administered as vapours or aerosols in order to deliver active substances that have a local or systemic effect. They can be liquids, semi-solids or solid preparations containing one or more active substances and usually also preservatives and excipients. During the inhaler development and manufacturing process, the effectiveness of the preservative must be demonstrated, as well as the uniformity of delivered dose, both for doses delivered by the same inhaler (intra-inhaler) and by different inhalers
(inter-inhaler). Today, we can characterise the major performance areas of inhalation and nasal aerosols, sprays, and powders related to dose delivery to the patient, including DDU and particle size, using our Next Generation Impactor from Copley and our Spraytec from Malvern, according to both the USP
<601>, <905>, <1601> and Ph.Eur. <0671>, Ph.Eur. <0523> for the following tests:
- Aerodynamic Particle / Droplet Size Distribution (APSD)
- Uniformity of delivered dose (DDU), intra-inhaler testing
- Aerodynamic assessment of nebulised aerosols
Other characterisations of the devices themselves (MDIs, PDIs, Nebulisers) according to both the USP <601>, <1601> and Ph.Eur. <2.9.18>, Ph.Eur. <2.9.44> for the following tests are also available in Les Ulis: fine particle dose, number of deliveries per inhaler, and leak rate. We also analyse all the different ingredients used within the inhalers, such as the active ingredients and excipients (e.g., propellant, solvent, diluent, antimicrobial preserving and solubilising agents). For this, Eurofins BioPharma Product Testing uses a broad range of equipment (e.g. GC-MS, ICP-MS, etc.) to perform:
- Uniformity of dosage unit, content, mass
- Residual solvents
- Elemental impurities
- Osmolality, pH, viscosity
- Reconstitution time (powder)
- Water content
- Clarity, colour, etc.
Contact us for further information or visit: www.eurofins. com/bpt
Eurofins BioPharma Services expands its biological sample storage services with the Hamilton BiOS XL4, offering clients unparalleled safety and security
Doug Irving, Director of Marketing, Eurofins Viracor Biopharma Services doug.irving@vbp.eurofins.com
 The biological samples gathered for clinical research are precious to pharmaceutical clients, and in most cases, irreplaceable. If these samples are compromised at any stage prior to analysis, the study results can be affected and potentially invalidated. Therefore, timely and appropriate handling and storage of these samples, with the ability to track the lifecycle factors for each one, is of paramount importance. So, it should come as no surprise that sample storage in regulated bioanalysis is one of the most critical aspects of clinical trial support.
The biological samples gathered for clinical research are precious to pharmaceutical clients, and in most cases, irreplaceable. If these samples are compromised at any stage prior to analysis, the study results can be affected and potentially invalidated. Therefore, timely and appropriate handling and storage of these samples, with the ability to track the lifecycle factors for each one, is of paramount importance. So, it should come as no surprise that sample storage in regulated bioanalysis is one of the most critical aspects of clinical trial support.
More than two years ago, as part of long-term planning for construction of a new facility, leaders at Eurofins Viracor BioPharma (EVB) evaluated their sample storage capabilities. They quickly recognised that the existing “farm” of 65 ultra-low temperature (ULT) freezers that are spread across multiple areas of the lab and used on a daily basis was not sustainable. The new facility would need to enable the BioPharma business to expand its storage services with existing customers and to acquire new customers that were looking for a full suite of sample management and storage offerings.
Ultimately, the decision was made to invest in a new system that would meet both clients’ needs and comply with the heightened regulatory requirements that are in effect for the storage of biological samples for clinical trial research. EVB chose the Hamilton BiOS XL4, a fully automated ultralow freezer system (-80°C) with triple redundant cooling, which would provide the ultimate in protection, reliability, operational efficiency, as well as ease of use. Installation of the new biostorage system began in 2022 and became fully operational this year. The new BiOS offers a fully automated record and a traceability system that runs on 21 CFR Part 11 compliant software (including automated barcode reading, full audit trail, and LIMS integration), enabling the team to improve the level of service offered to customers, while also increasing the efficiency of storage operations.
The integration of the BiOS into sample processing also offers a significant competitive advantage in the industry through higher volume storage capacity and sample processing throughput. The increased safety and security that the system offers further enhances EVB’s reputation as experts in the handling of all types of biological samples for pharmaceutical research. For more information visit: www.eurofins-viracor.com/biopharma/services/client-support/sample-management-and-biostorage/