Advancing weight-loss therapeutic discovery through integrated science
Carol Austin, PhD, Sr. Director, Drug Discovery Project Management and IDD Business Line Leader, Eurofins Discovery, carol.austin@discovery.eurofinsus.com

With global obesity rates projected to surpass one billion by 2030, the world faces a mounting health and economic burden that demands urgent scientific breakthroughs. Addressing this challenge requires collaborative efforts and robust scientific infrastructure to support the development of effective weight-loss therapies.
Eurofins Discovery contributes to this effort by providing fully integrated drug discovery solutions grounded in deep scientific expertise. Our capabilities span the entire discovery continuum from target identification and hit finding to lead optimisation and in vivo validation – enabling data-driven decision-making and streamlined development processes.
A key resource in our metabolic research portfolio is the obesityLITE™ panel, a suite of more than 25 cell-based assays targeting receptors, such as GLP-1R, GIPR, GCGR, and MC4R. These assays support rapid screening of mono, dual, and triagonist therapies, offering insights into potency, selectivity, mechanism-of-action, and ligand imbalance within a single workflow.
Our innovative assays have been widely adopted across the industry, contributing to the development of many weight-loss drugs currently on the market or in clinical trials. Their inclusion in regulatory filings, peer-reviewed publications, and patents highlights their scientific and regulatory relevance.
In addition, our portfolio includes over 10,000 off-the-shelf assays for GPCRs, nuclear hormone receptors, and metabolic enzymes, covering a broad range of next-generation therapeutic targets. The BioMAP® human primary cell platform further enhances translational relevance by modelling disease biology to support mode-of-action studies, toxicity profiling, and benchmarking.
For in vivo studies, Eurofins Discovery supports programmes with clinically relevant models for type 2 diabetes, diet-induced obesity, and MASH, including a humanised liver model for robust preclinical validation.
With a global infrastructure and a collaborative approach, Eurofins Discovery supports the scientific community in advancing the future of weight-loss therapeutics.
Environmental monitoring services expand, spanning North America and offering regional accessibility with personalised solutions
Tom Lehman, PhD, Vice President, Microbiology, Mycoplasma Testing, Extractables and Leachables, thomas.lehman@bpt.eurofinsus.com

To ensure that manufacturing facilities are established and maintained under strict contamination control strategies, conducting an Environmental Monitoring Performance Qualification (EMPQ) and developing a subsequent Environmental Monitoring (EM) program are essential. Designing an effective EMPQ and EM strategy requires a thorough understanding of regulatory expectations as well as hands-on experience. Eurofins BPT’s expertise in delivering these services to clients throughout the Biopharmaceutical industry are well-revered, data-trusted solutions, now with more geographic accessibility.
Eurofins BioPharma Product Testing (BPT) is recognised in the industry for providing high quality data and a comprehensive services portfolio with deep scientific expertise. We are committed to helping clients bring their products to market efficiently and confidently.
Eurofins BPT has created a broad service offering for EMPQ and EM throughout North America. With nine sites spanning from the East Coast to the West Coast and into Eastern Canada, we provide regional accessibility and personalised support tailored to our clients’ specific needs. Our team utilises standardised equipment to perform the following testing:
- Viable and non-viable air sampling
- Surface contact plates and swabs
- Testing of compressed gases (viable, non-viable, dew point, hydrocarbon/oil mist)
- Water collection and testing (source, purified, WFI, clean steam)
- Total aerobic microbial count
- Total coliform/fecal coliform
- Endotoxin
- TOC
- Conductivity
- Nitrate
- Heavy metals
With more than 25 years of industry experience, Eurofins BPT is a trusted partner in delivering reliable, regulatory-compliant EMPQ and EM services. Our extensive North American network, standardised methodologies, and expert teams ensure consistent, high-quality results tailored to your facility’s unique needs.
Whether you are establishing a new cleanroom or maintaining ongoing compliance, Eurofins BPT is here to support your success every step of the way.
Contact Us today to learn how our EMPQ and EM solutions can help you maintain a state of control and accelerate your path to market.
Eurofins BPT Italy site offers enhanced access to viral clearance studies for European clients
Giulia Sbarufatti, Business Unit Manager Virus Testing (IT), Eurofins Bio-Pharma Product Testing Italy, Giulia.Sbarufatti@bpt.eurofinseu.com

Eurofins BioPharma Product Testing (BPT) Italy has officially launched Viral Clearance Services at its Milan site, further expanding the Eurofins BPT network’s global footprint in viral safety services.
Since 2009, the Virus Testing Unit in Milan has supported biopharmaceutical companies with viral inactivation studies (e.g.,low pH and solvent/detergent treatments) on their products. In response to the growing development of biologic drugs and increasing awareness of viral safety requirements, the expansion into viral removal studies represents a significant enhancement of our analytical capabilities. This milestone strengthens both Eurofins BPT’s global offering and its local presence in Europe.
What differentiates us is our flexible, client-focused approach—each study is tailored to meet the specific needs and expectations of our clients. Additionally, our reduced backlog allows for faster project initiation, offering a critical time advantage in development timelines.
Clients can choose between two service models:
- A standard full-service model, in which Eurofins BPT conducts the entire study, including technology transfer and scaled-down process execution with virus spiking and detection.
- A hybrid-service model, where clients access our virus testing facility and use our purification equipment (e.g., ÄKTA systems or filtration setups), while our expert Virology Team manages all virological operations, including spiking, sampling, and virus detection.
The Virus Testing Unit operates within a GLP-compliant testing facility, supported by a comprehensive viral library (over 40 strains, including XMuLV, PRV, MVM, and Reo-3), a 450 m² laboratory, an ÄKTA Pure 25 system, and all necessary equipment for viral clearance studies.
For further information and details, please visit: www.eurofins.com/biopharma-services/product-testing/services/biopharma-product-testing-services/process-validation/viral-clearance/
Improving potency assays through semi-automation
Frances Reichert, PhD, Biologics Technical Specialist Biologics, Eurofins BioPharma Product Testing Munich GmbH, frances.reichert@bpt.eurofinseu.com

As specified in the ICH Q6B guideline, potency is a critical quality attribute for biopharmaceuticals, playing a key role in quality control to ensure product safety and efficacy. Regulatory agencies emphasise that potency assays must align with a drug’s mode of action to provide physiologically relevant results. Although cell-based assays are commonly used for this purpose, they are prone to variability due to the use of living cells, assay complexity, and analyst experience, making it difficult to achieve consistent and reproducible results.
While full end-to-end automation in a GMP-regulated environment remains challenging, a modular semi-automated approach offers a highly flexible and cost-effective solution. This strategy enables increased precision and reduced variability while supporting adaptable workflows. At Eurofins BioPharma Product Testing Munich, we use semi-automated systems in our laboratories, including the recently GMP-validated Hamilton Microlab® STAR liquid handler and Integra platforms such as the VIAFLO96 and ASSIST PLUS to support method development, qualification/validation, and GMP sample routine testing.
In a case study, we used the cAMP Hunter™ Bioassay Kit to evaluate the potency of a peptide agonist and demonstrate the benefits of semi-automation. Initially, the semi-automated method using the Integra VIAFLO96 pipette showed high replicate variability and overestimated potency values compared to manual performance. However, after optimising pipetting speeds and reagent additions, the results became much more consistent, with potency recoveries of 103% and 93%, closely aligning with the expected value of 100%. This improved precision and accuracy was confirmed across the method range of 50–200%.
Semi-automation is a valuable tool for potency assays, increasing throughput and reducing analyst time without compromising accuracy or precision. However, careful optimisation of automation methods is essential to ensure effective implementation and results comparable to those achieved manually. Overall, these technologies can improve method precision and consistency, increase throughput, and reduce manual handling errors. For more information, visit: www.eurofins.de/biopharma-product-testing/biopharma-product-testing-services/large-molecules-biologics/automation-of-analytical-procedures/
Eurofins BPT Italy delivers rapid sterility test results in four days
Sara Baroni, Eurofins BioPharma Product Testing Italy, Sara.Baroni@bpt.eurofinseu.com; Silvia Scotti, Eurofins BioPharma Product Testing Italy, Silvia.Scotti@bpt.eurofinseu.com

In recent years, the regulatory landscape has encouraged the adoption of rapid microbiological methods as alternatives to traditional sterility compendial tests, which currently require a minimum incubation time of 14 days. General chapters 5.1.6 of the European Pharmacopoeia and <1223> of the USP provide clear guidelines on the implementation of alternative methods, emphasising the possibility of selecting the most suitable technology based on the product type, provided it is properly validated and demonstrated to be comparable to traditional methods.
In response to this regulatory evolution and the growing demand to reduce release times, benefiting both patient safety and manufacturing companies, Eurofins BioPharma Product Testing (BPT) Italy has adopted a multi-technological approach, integrating rapid and automated methods to support clients in choosing the most suitable solution.
In addition to the classic compendial sterility test Ph. Eur. 2.6.1/USP <71>, Eurofins BPT Italy now offers the following innovative technologies in compliance with 21 CFR Part 11 and data integrity:
- RedOne® by Redberry (based on Solid Phase Cytometry): sterility test in four days. With high sensitivity and automation, it supports early decision-making in batch release processes.
- Celsis® by Charles River (based on ATP bioluminescence): rapid detection through ATP bioluminescence. This allows product release in a minimum of six days with significant optimisations for large sets of samples.
- BacT/ALERT 3D: automated test for cellular products, validated according to the specific chapter of the pharmacopoeia EP 2.6.27. It’s suitable for cell-based preparations with high complexity or reduced shelf life.
The adoption of rapid and automated microbiological methods drastically reduces the time-to-results, prevents production blocks, optimises deviation management, and improves timely patient access to advanced therapies.
Eurofins BPT Italy is a strategic partner in the implementation of innovative solutions, in line with the principles of Quality by Design and Process Analytical Technology, promoted by the FDA and EMA. For more information visit: www.eurofins.com/biopharma-services/product-testing/services/biopharma-product-testing-services/microbiology/rapid-sterility-testing/
Sustainability. Efficiency. Consistency.
Tessa Patton, Manager, Bio/Pharmaceutical Microbiology, Eurofins BioPharma Product Testing in Lancaster, PA, Tessa.Patton@bpt.eurofinsus.com
 The words sustainability, efficiency, and consistency, don’t immediately bring bacterial endotoxin testing to mind. Bacterial endotoxin testing (BET) can feel like anything but sustainable, efficient, or consistent, but new technologies open a whole new arena in BET.
The words sustainability, efficiency, and consistency, don’t immediately bring bacterial endotoxin testing to mind. Bacterial endotoxin testing (BET) can feel like anything but sustainable, efficient, or consistent, but new technologies open a whole new arena in BET.
In the complex world of biopharmaceutical quality control, BET plays a critical role in ensuring the safety of drugs, vaccines, and medical devices. The Eurofins Bio-Pharma Product Testing (BPT) team is proud to onboard new technologies and practical solutions to deal with the challenges of BET. Onboarding bioMérieux’s Recombinant Factor C (rFC) reagent and the Sievers Eclipse™ Bacterial Endotoxin Testing Platform for kinetic chromogenic testing, demonstrates our commitment to sustainability, workflow efficiencies, and data consistency.
bioMérieux’s rFC, a part of their ENDONEXT™ technology, is a nonanimal-derived alternative to the traditional limulus amebocyte lysate (LAL). rFC mimics the natural Factor C found in horseshoe crab blood and binds specifically to endotoxin to trigger a cascade reaction, resulting in a measurable signal (either fluorescence or luminescence).
This synthetic reagent reduces lot-to-lot variability, resulting in a more consistent performance. Eurofins BPT selected bioMérieux’s ENDOZYME® II GOPLATE™ technology to improve both consistency and workflow efficiency. This aids in reducing analyst time and eliminates analyst-to-analyst technique variability. It’s also compliant with USP <86> and Ph. Eur.2.6.32.
The Sievers Eclipse Bacterial Endotoxin Testing Platform significantly reduces the use of LAL by up to 90% as compared to traditional BET methods. Similar to the ENDOZYME II GOPLATE, the Eclipse utilises a pre-loaded microplate, again reducing hands-on time and eliminating technique variability. It does all of this while maintaining compliance to USP <85>, Ph. Eur. 2.6.14, and JP 4.01.
Combining these innovations with our expertise has meaningful impact on the industry and the environment. As the biopharmaceutical industry continues to evolve, adopting and developing technologies to increase efficiencies, reduce variability, and support sustainability is vital. For more information, visit: Sustainable rFC Endotoxin Testing | Eurofins BPT.
In Vitro Safety Insight: an automated visualisation tool for early target-adverse event identification in secondary pharmacology to support early drug safety assessment
Emilie Desfosses, Preclinical Safety Director, Eurofins Cerep (Eurofins Discovery Company), emilie.desfosses@discovery.eurofinseu.com
Secondary pharmacology studies are typically included in Investigational New Drug (IND) applications. They help to characterise the off-target activity risks of drug candidates, ideally, early in the drug discovery process from the lead generation stage and contribute to their safety evaluation. It is well established that activity at certain off-targets can be associated with adverse events (AEs) in humans, and that reducing target promiscuity may help limit the occurrence of drug-related AEs (Brennan et al., 2024, ICH guideline S7A). However, despite the availability of extensive literature and public databases, understanding and predicting potential AEs linked to target modulation remains a significant challenge.
With the increasing number of targets requiring screening, computational approaches are becoming an attractive solution. In Vitro Safety Insight is a computational tool developed by Eurofins Discovery to support the interpretation of secondary pharmacology data from SafetyScreenTM panels, including the latest recommended targets (SafetyScreen77) from the leading pharmaceutical industries (Brennan et al.,2024)
The tool includes:
- A spatial overview and heatmap,focusing on the pharmacological data, enabling easier comparison between compounds and ranking.
- A custom hazard metric to assess organs at risk based on the compound’s pharmacological profile. This scoring system combines pharmacological data with target–adverse event associations derived from our database, which is built through a semi-automated process, integrating both public and proprietary sources.
- Suggested follow-up assays according to the organ system at risk to build a derisking strategy.
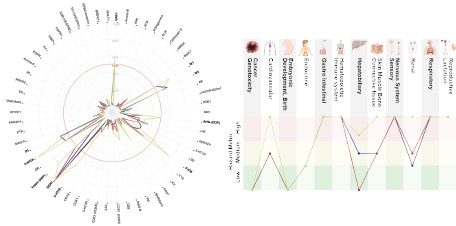
Figure 1 below: Secondary pharmacology data visualisation and interpretation figures displaying Organ System Hazard Metric Profile (OSHMP).
- A. Pharmacology data presented on a radar plot displaying inhibition percentage of the reference activity by the tested compounds (10 μM) on the target-based assays activity (binding and enzymatic). Target names in bold are those showing inhibition percentage higher than 50%, indicating a hit.
- B. Interpretation figures displayed as an OSHMP for tested SSRI compounds.
 At Eurofins Discovery, we are committed to developing the most updated solutions for data-driven decision-making in support of human safety. For accurate risk assessment, the tool will be updated to a better version with more details on AEs related to each target and will require translational quantitative information (potency and plasmatic exposure) and contextualisation to refine interpretations and adapt risk mitigation strategies. For more information, visit: www.eurofinsdiscovery.com/solution/in-vitro-safety-insight
At Eurofins Discovery, we are committed to developing the most updated solutions for data-driven decision-making in support of human safety. For accurate risk assessment, the tool will be updated to a better version with more details on AEs related to each target and will require translational quantitative information (potency and plasmatic exposure) and contextualisation to refine interpretations and adapt risk mitigation strategies. For more information, visit: www.eurofinsdiscovery.com/solution/in-vitro-safety-insight
References: 1.Bowes, J., Brown, A. J., Hamon, J., Jarolimek, W., Sridhar, A., Waldron, G., & Whitebread, S. (2012). Reducing safety-related drug attrition: The use of in vitro pharmacological profiling. Nature Reviews Drug Discovery, 11(12), 909–922. https://doi.org/10.1038/nrd3845 2. Brennan, R. J., Jenkinson, S., Brown, A., Delaunois, A., Dumotier, B., Pannirselvam, M., Rao, M., Ribeiro, L. R., Schmidt, F., Sibony, A., Timsit, Y., Sales, V. T., Armstrong, D., Lagrutta, A., Mittlestadt, S. W., Naven, R., Peri, R., Roberts, S., Vergis, J. M., & Valentin, J.-P. (2024). The state of the art in secondary pharmacology and its impact on the safety of new medicines. Nature Reviews Drug Discovery. https://doi.org/10.1038/s41573-024-00942-3 3. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. (2000). ICH S7A: Safety pharmacology studies for human pharmaceuticals. https://database.ich.org/sites/default/files/S7A_Guideline.pdf 4. https://emea.eurofinsdiscovery.com/solution/in-vitro-safety-insight
The power of SPR Biacore™ and BLI Octet® in QC GMP methods
Frances Reichert, PhD, Biologics Technical Specialist Biologics, Eurofins BioPharma Product Testing Munich GmbH, frances.reichert@bpt.eurofinseu.com
 Surface Plasmon Resonance (SPR) using the Biacore™ (Cytiva) platform and Bio-Layer Interferometry (BLI) using the Octet® (Sartorius AG) platform are powerful, label-free, optical biosensor technologies that produce real-time analysis of biomolecular interactions. Unlike traditional methods, such as ELISA, both SPR and BLI eliminate the need for labelling with fluorescent or enzymatic tags, allowing the direct assessment of kinetic parameters and binding affinity with high sensitivity and reproducibility.
Surface Plasmon Resonance (SPR) using the Biacore™ (Cytiva) platform and Bio-Layer Interferometry (BLI) using the Octet® (Sartorius AG) platform are powerful, label-free, optical biosensor technologies that produce real-time analysis of biomolecular interactions. Unlike traditional methods, such as ELISA, both SPR and BLI eliminate the need for labelling with fluorescent or enzymatic tags, allowing the direct assessment of kinetic parameters and binding affinity with high sensitivity and reproducibility.
In a comparative case study, assays were established on both platforms using the same ligand and a therapeutic monoclonal antibody as analyte. The reference standard representing 100% nominal potency was included in both setups.
In the first assay, varying concentrations of the analyte were evaluated for binding to human recombinant CD32 ligand. A linear regression model was fitted using PLA 3.0 software (Stegmann Systems) to determine the relative potency.
In the second assay, the association and dissociation of the analyte with the recombinant CD16a ligand were assessed using various concentrations of the reference standard and the sample. Binding affinities (KD values) were then calculated using instrument-specific software.
Both technologies demonstrated high accuracy and precision in determining relative potency and binding affinities. This provides major advantages over label-based technologies such as ELISA. When comparing both technologies we found that SPR was more precise than BLI, making it particularly well-suited for applications requiring detailed kinetic resolution. In contrast, BLI technology has a shorter assay run time, making it advantageous for high-throughput screening workflows.
In summary, both SPR and BLI offer distinct benefits. Based on our experience, the rapid assay format of BLI is ideal for screening applications, while SPR provides precise kinetic and affinity data, and in some cases, higher sensitivity. Both technologies represent robust, label-free alternatives to traditional endpoint assays, enhancing the reliability and efficiency of biopharmaceutical characterisation and potency assessment. For more information, visit: www.eurofins.de/advanced-spr-biacore-and-bli-octet-services-for-biopharmaceutical-analysis.pdf